What Does “Amphoteric” Mean in Chemistry?
Let us refresh your memory by explaining what “amphoteric” means. Oxides are classified into acidic, basic, neutral, amphoteric, peroxides, mixed, and superoxides. These classifications are drawn from an oxide’s ability to react with acids or bases or its oxygen atom’s oxidation number.
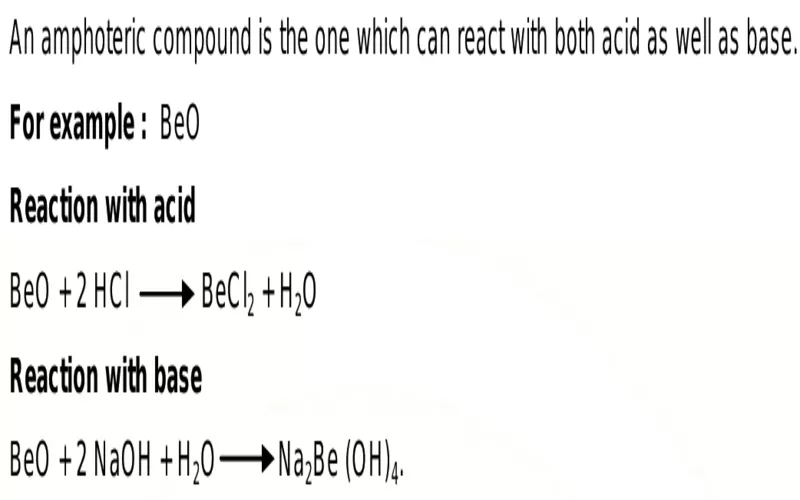
Using this template, an oxide is amphoteric when it can react with acids and bases. When it reacts with a base (or is in a basic condition), it becomes an acid, producing salt and water. In an acidic condition, however, it acts as a base, often producing a complex salt. In some cases, as seen in aluminum and zinc oxides, it can form salt and water.
Is Beryllium Oxide Amphoteric?

Yes, Beryllium oxide is amphoteric. Consequently, in the presence of a base, it becomes the acid. Likewise, when it reacts with an acid, it becomes the base. But how does it achieve its amphoteric properties? Let us explain to the letter in this section.
A simple way to show that beryllium oxide is amphoteric is to use its metallic nature and ability to form covalent bonds. Metallic oxides have basic properties. Beryllium belongs to Group II (alkali earth metal). Although it is not a typical metal like its Group II members, e.g., potassium and magnesium, it is basic. Hence, in the presence of acids, it acts as the base.
Even though Beryllium is a metal, unlike other Group I and II members, it does not form only ionic bonds. The beryllium ion (Be2+) has a small size and high charge density, making it more covalent. As a result, it can act as an acid, forming a bond with nucleophiles like hydroxide ions.
Another way to explain why beryllium oxide is amphoteric is to use electronegativity (the ability of an atom to attract electrons to itself). An electronegativity difference between an element X and oxygen > 2 means the element’s oxide can easily give off its oxygen. A difference <1 means the oxide won’t.
Beryllium and Oxygen have an electronegativity difference between 1 and 2. Hence, the reaction condition will determine whether the oxygen easily leaves or not, i.e., becomes an amphoteric region. In acidic conditions, beryllium oxide gives off its oxygen to the proton (H+) to form water, itself forming the corresponding salt. However, in basic conditions, it forms a complex.
How Beryllium Oxide Act as a Base
In acidic conditions, beryllium oxide acts as a base. It easily gives off its oxygen, which will accept the protons (H⁺) from the acid. The oxygen atom can do this because it is electron-rich due to its higher electronegativity than the proton (H+).
A summary of the process:
-
An acid HX dissociates into H+ and X-
-
The proton (H+) combines with O2−, forming water.
-
The beryllium ion Be2+ combines with X, forming BeX.
Using hydrochloric acid (HCl), the electron-rich oxygen will accept the proton (H+) to form water. On the other hand, the Beryllium ion will accept the chloride ion, forming beryllium chloride.
BeO+2HCl→BeCl2+H2O
Using sulfuric acid (H2SO4), the electron-rich oxygen will accept the proton (H+) to form water. The Beryllium ion will accept the sulfate ion, forming beryllium sulfate.
BeO+H2SO4→BeSO4+H2O
In this case, the last example is nitric acid (HNO3). Although you might think that HNO3’s high oxidizing power will have some effect on BeO, that would be wrong. Beryllium oxide is stable, and the reaction goes as the other two. The oxygen ion will accept the proton (H+) to form water, and the Beryllium ion will combine with the nitrate ion, forming beryllium nitrate.
BeO+2HNO3→Be(NO3)2+H2O
How Beryllium Oxide Act as An Acid
In a basic condition, beryllium oxide becomes an acid. The beryllium ion’s lower electronegativity and partial positivity make it an electrophile. As a result, it can combine with nucleophiles, such as hydroxide ions (OH-), to form a complex salt.
The most common example is the reaction between sodium hydroxide and beryllium oxide. Beryllium oxide will form a potassium berrylate complex in the presence of NaOH.
BeO+2NaOH+H2O→Na2Be(OH)4
Another example of a base you can react with beryllium oxide is potassium hydroxide (KOH). The oxide will form a soluble complex salt called potassium berryllate
BeO+2KOH+2H2O→K2[Be(OH)4]
Can Beryllium Oxide React With Weak Acids and Bases
Yes, beryllium oxide is amphoteric and will react with weak acids and bases follows the previous explanation. However, these reactions require certain tweaks to form the expected products while being fast.
For example, the reaction between Beryllium Oxide and acetic acid (CH3COOH) is slow and less vigorous due to the acid’s low dissociation constant. Nevertheless, the reaction will proceed and yield beryllium acetate and water.
BeO+2CH3COOH→(CH3COO)2Be+H2O
In the case of weak bases, the Beryllium oxide and ammonia (NH₃) reaction might require heat. Nevertheless, it will form a soluble beryllium-ammonia complex. However, the reaction is not fast compared to a strong base like sodium hydroxide.
BeO + 2NH3 +H20 → [Be(NH3)2(OH)2]
The reaction is also less pronounced with ammonium hydroxide. Nevertheless, BeO still exhibits its amphoteric property, forming a beryllium hydroxide complex ion.
BeO+NH4OH+H2O→[Be(OH)3]−+NH4+
The reaction speed (either weak acid or base) will depend on the degree of dissociation of the acid or bases. The fewer the reactive ions (H+ or OH−), the slower the reaction and the amount of products formed.
Bottom Line
You have the answer to your question. If you wonder if beryllium oxide is amphoteric, i.e., acidic and basic, it is. Beryllium oxide is amphoteric, just like other elements like aluminum, lead, and zinc. This means it can either be an acid or base in the right condition.