
Since surface atoms are susceptible to oxidation under certain conditions, corrosion can degrade materials through chemical interactions. Sometimes, corrosion protects the material but can lead to discoloration, reduced structural integrity, and a shortened service life. Therefore, this article will help you get up to speed on the different types of corrosion and their effects on various materials.
Corrosion is a very complex electrochemical process that affects materials in all industries. This guide will take you through the basic principles of corrosion, its different types and effective prevention strategies using modern corrosion protection materials.
Quick Links
- Why Do Materials Corrode?
- Can Most Victims Of Corrosion Be Metalheads?
- Basic Factors Affecting Corrosion
- Corrosion Type
- Corrosion Resistant Materials Selection Guide
Why Do Materials Corrode?
Corrosion is the natural degradation process of materials (especially metals) through chemical or electrochemical reactions with the environment. When the surface atoms of a material come into contact with elements in the environment (such as oxygen and moisture in space), they may react and transform into some unwanted substances, resulting in a degradation of the basic properties of the material.
While all materials are subject to some form of degradation, metals are more susceptible to corrosion due to their unique electrochemical properties. Metals corrode naturally, and they corrode faster in certain environments (such as when exposed to air and water). Of course, there are some relatively stable metals, such as gold and platinum, which are not easy to rust and corrode due to their unique chemical properties.
Can Most Victims Of Corrosion Be Metalheads?
Generally, metals will corrode quickly in certain environments due to their unstable nature. In particular, some metals in the more reactive areas (such as zinc, iron, etc.) are more susceptible to corrosion, while some metals in the less reactive areas (such as platinum, gold, etc.) are not easily corroded. Usually we refer to corrosion of metals as rust.
Most corrosion will damage the material itself, but what is interesting is that when aluminum oxidation is corroded, the oxide produced will form a dense oxide film. This oxide film can in turn protect the aluminum metal inside the film from being damaged. This is followed by oxidation, which shows that certain materials can form beneficial passivation films.
Non-metallic materials can also corrode because they have strong ionic and covalent bonds and few free atoms. Generally speaking, non-metallic materials such as plastic materials, ceramic materials and polymers will gradually be corroded and decomposed when exposed to sunlight for a long time.
Basic Factors Affecting Corrosion
Are you curious about what are the basic factors affecting corrosion?
Environmental Factors
Temperature:Temperature plays a critical role in the corrosion rate, especially in some petrochemical plants, refineries and aerospace industries where high temperatures can accelerate the corrosion of materials. Therefore, in these fields, ceramic materials are usually chosen, which have stronger high temperature resistance and corrosion resistance.
Humidity, atmosphere:In addition to temperature, moisture and atmospheric conditions are also key factors affecting corrosion. They create an ideal environment and conditions for corrosion and can carry out a series of electrochemical reactions. When metal materials are exposed to areas with a lot of moisture, they are likely to develop different types of rust. The main reason why this happens is that moisture accelerates oxidation and corrosion. Therefore, in a dry or oxygen-free environment, the rate of metal corrosion can be effectively slowed down.
Material Properties
Active Metals:The electrode potential of different metals affects the corrosion resistance of the metal. Metals with higher electrode potential have better corrosion resistance than metals with lower electrode potential. Some active metals such as zinc and iron are very prone to corrosion.
Impurities:In addition to the properties of the material itself, the presence of impurities will also affect the corrosion rate. If impurities such as salt are attached to the surface, the corrosion will be accelerated. If a natural oxide layer like aluminum is attached, the corrosion efficiency will be greatly reduced.
Corrosion Type
So, what are the types of corrosion? We have sorted out 9 different types of corrosion for you to help you quickly understand.
Galvanic Corrosion
Galvanic corrosion is a corrosion phenomenon caused by electrochemical reaction when two different metal materials come into contact in an electrolyte environment. The essence of this corrosion is that a primary battery is formed between the two metals, in which the more active metal is the anode and will be corroded first, and the more inert metal is the cathode and will be protected, and the corrosion will be slowed down or even stopped.
The mechanism of galvanic corrosion is that when two metals come into contact in an electrolyte solution (such as seawater), the anode metal loses electrons and forms metal ions, and the electrons flow to the cathode, causing a reduction reaction (such as oxygen reduction and water decomposition). Galvanic corrosion will cause the corrosion of the anode metal to continue to intensify.
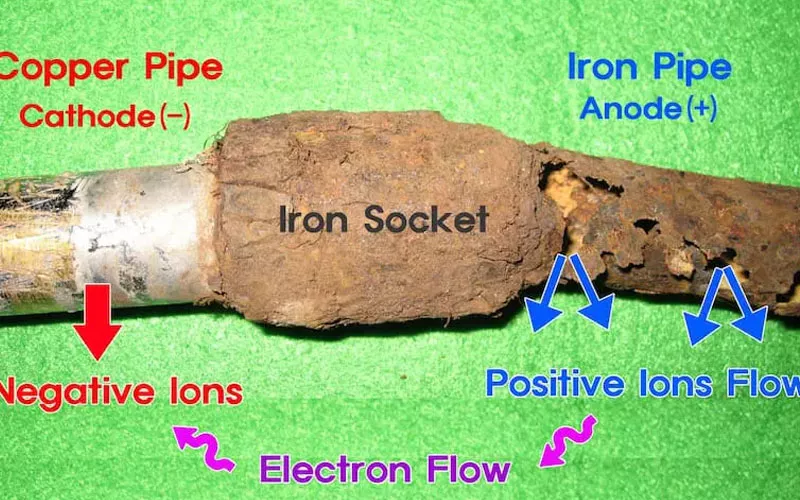
Galvanic corrosion is common in marine environments, battery terminals, and building pipelines, and is a more serious corrosion.
- Marine Environment:Seawater is a naturally highly conductive electrolyte, so galvanic corrosion is common in marine engineering. When some hull metal (usually steel) comes into contact with the propeller (usually copper alloy), if no protective measures are taken, it will continue to be corroded until it is scrapped.
- Battery Terminals:The positive and negative terminals of the battery and the connectors are usually made of different metals. When the two come into contact, oxides or other corrosion products will be generated on the surface of the terminals due to electrochemical reactions, resulting in increased resistance and affecting the overall efficiency of the battery.
- Building And Plumbing:Pipes are often located in a sewage environment with high humidity. When different metals are connected and contacted, galvanic corrosion is likely to occur, such as the joints between steel pipes and copper pipes.
So how to prevent galvanic corrosion? Common measures are as follows:
- Sacrificial Anode Protection method:Choose a more active metal as the sacrificial anode to make it corrode first, so as to protect the main building metal structure from damage. This method is often used in ships, sea platforms, etc.
Choose Metals That Are Close To The Electrodes:When designing, try to choose a combination of two metals with a small difference in electrode potential, preferably within 0.2V. For example, choose a combination of copper alloy and brass at the connection of a water pipe.
Use a protective layer to isolate electrical contact: Add an isolation layer between the two metals to prevent contact, such as epoxy resin, ceramic coating, insulating gasket, etc.
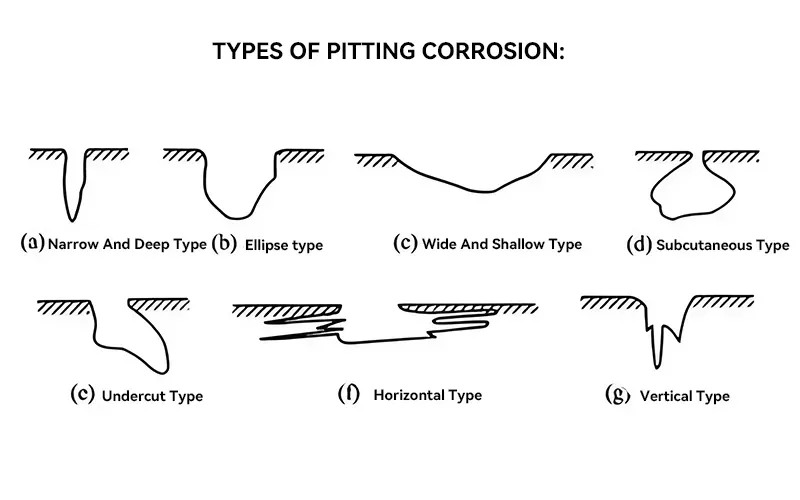
Pitting Corrosion
Pitting is a very hidden form of corrosion that often forms local holes in metal. This type of corrosion is highly destructive and dangerous. It can penetrate deeply but cause minimal damage to the surface. Once corrosion begins, it will continue to self-catalyze and is difficult to detect before major damage occurs.

The characteristics and destructiveness of pitting corrosion are mainly as follows:
- Hiddenness:The surface damage caused by pitting is usually small and difficult to detect with the naked eye or routine inspection in the early stages. However, the internal corrosion may have penetrated deep into the metal structure and may have destroyed or even perforated the metal structure by the time it is discovered.
- Autocatalytic Property:A closed corrosion environment will be formed in the pits formed by pitting corrosion, which will continue to autocatalyze, leading to accelerated corrosion.
- Locality:Pitting often occurs in areas where the metal surface is locally passivated or uneven, such as scratches, depressions, cracks, etc.
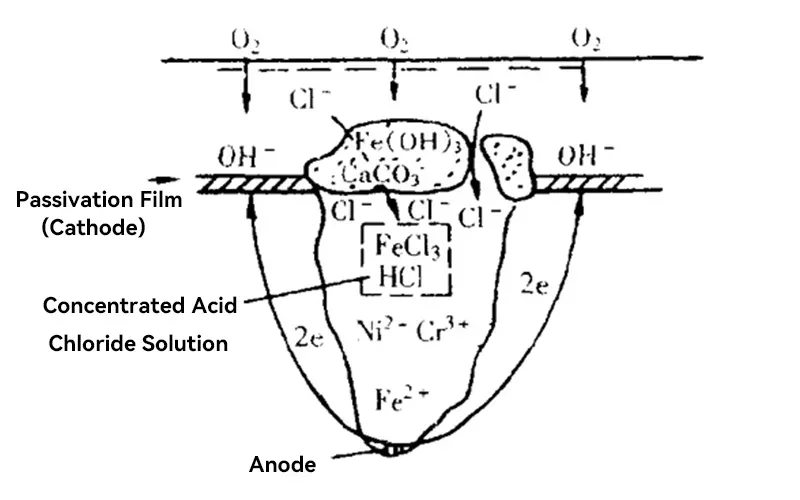
The main factors causing pitting are as follows:
- Destruction Of Passivation Film:When some of the passive films protecting the internal metal are partially damaged, it is likely to become the starting point of pitting corrosion.
- Metal Material Defects:Grain boundaries, inclusions, uneven structures or welded joints inside the metal are likely to be sensitive parts to pitting corrosion.
- Environment:When in a humid, high chloride, high temperature environment for a long time, the probability of pitting corrosion will greatly occur.
Pitting corrosion is very harmful. Even stainless steel, a material with excellent corrosion resistance, is prone to pitting corrosion in a high concentration of chlorine environment, such as seawater or hot and humid industrial equipment. Therefore, it is extremely important to prevent pitting corrosion. Common preventive measures are as follows::
- The metal is polished internally to prevent local defects and an anti-corrosion coating is used for additional protection
- Use pitting-resistant metals or alloy materials such as stainless steel containing molybdenum (Mo) or high chromium (Cr) content. In extreme environments, more corrosion-resistant super alloys can also be used.
- Reduce the concentration of chloride ions in the environment and desalinate seawater
- Regular maintenance inspections to prevent corrosion conditions from worsening
High Temperature Corrosion
This type of corrosion occurs in high temperature environments above 400°C (750°F). High temperature corrosion is particularly common in industrial environments such as gas turbines, furnaces, power plants, and manufacturing facilities.

The combination of extreme temperatures and corrosive gases creates particularly aggressive conditions that can quickly corrode some high-performance alloys. Moreover, the molten salt and ash deposition left by atmospheric pollutants will also accelerate corrosion. Therefore, high heat-resistant alloys and complete cooling mechanisms are crucial to prevent high-temperature corrosion.
Intergranular Corrosion
Metals are composed of grains, with atoms arranged in an orderly manner within each grain. Corrosion affects the grain boundaries because this area is more reactive than the matrix. Intergranular corrosion is mainly caused by impurities at the grain boundaries and the enrichment or depletion of alloying elements in the boundary area.

Intergranular corrosion is a common localized corrosion phenomenon, which mainly occurs in the grain boundary area of metal materials. Many metals are usually composed of many grains, and the atomic arrangement inside each grain is orderly, but at the junction of the grains, that is, the grain boundary area, the atomic arrangement is more disordered and the energy is higher, so this area is more susceptible to corrosion.
The main reason for intergranular corrosion is the presence of some special chemical components in the grain boundary area, which leads to distribution differences. If there is an accumulation of impurities near the grain boundary, the presence of these impurities will reduce the corrosion resistance of the grain boundary, making it more susceptible to corrosion.
In addition, if certain alloying elements are enriched or depleted at the grain boundaries, the electrochemical characteristics at the grain boundaries will change, leading to increased corrosion. In industry, during the heat treatment or welding of stainless steel, if the chromium content at the grain boundaries is reduced due to the precipitation of chromium carbide, a chromium-depleted zone will be formed, which will lead to intergranular corrosion.
Intergranular corrosion not only weakens the mechanical strength of metals, but can also cause the entire metal structure to fail, so it needs to be protected. The most common means of protection is to use appropriate heat treatment processes, reduce impurities in the material, and select alloy materials containing stabilizing elements (titanium or niobium).
Environmental Cracking
Environmental cracking is caused by various environmental factors, such as chemical, stress and temperature.
- Stress Corrosion Cracking(SCC)SCC represents a dangerous combination of mechanical stress and a corrosive environment. Stress corrosion is the most dangerous of all types of corrosion because it can cause materials to fail without warning.

Stress corrosion occurs when some materials are subjected to tensile stress in a corrosive environment and extreme heat. Stress corrosion occurs when metal expands and contracts due to temperature changes, which weakens the integrity of the metal structure.
Stress corrosion occurs when tiny cracks first appear on the metal surface. Over time, the cracks will gradually expand and cause structural damage. This type of corrosion occurs when stainless steel is subjected to stress in a chloride environment.
The main preventive measures against stress corrosion are::
- Choose materials carefully
- Stress relief treatment
- Environmental Control Measures
- Fatigue CorrosionLike stress corrosion, fatigue corrosion can also cause premature cracking of metals in corrosive environments. Fatigue corrosion destroys protective layers and accelerates the corrosion process. Of course, fatigue corrosion can be controlled by reducing or eliminating cyclic stresses and avoiding vibration transmission designs.
Uniform Corrosion
This type of corrosion generally occurs on the surface of metals, and the lack of a protective layer is the main cause of this type of corrosion. When chemical or electrochemical reactions occur evenly across the entire metal surface, the metal becomes thinner and weaker.

This type of corrosion is easily observed and causes little damage to the performance of the metal. Common aluminum, zinc, iron, steel and lead will corrode uniformly when continuously exposed to a corrosive environment.
Microbiological Corrosion
Microbiologically Influenced Corrosion (MIC) is a special type of corrosion caused by chemoautotrophic microorganisms. These microorganisms include bacteria, algae and fungi, which can colonize metal and non-metal surfaces and accelerate the corrosion process through metabolic activities.

This type of corrosion can occur in a variety of environments, most commonly in the ocean, oil, gas pipelines, and wastewater treatment equipment. It is an important reason for the sudden failure of many industrial equipment.
The mechanism of microbial corrosion varies greatly depending on the microbial community and environment, such as:
Sulfate-Reducing Bacteria(SRB):This bacteria mainly reduces sulfate to hydrogen sulfide (H2S), which reacts with metals and causes corrosion. It may cause severe pitting and intergranular corrosion.
Acid-producing Bacteria:The metabolism of some microorganisms produces organic or inorganic acids, which are likely to corrode materials.
Iron-oxidizing And Iron-Reducing Bacteria:These bacteria will change the electrochemical conditions on the metal surface, exacerbating the corrosion process.
Adhesive Biofilm:Some microorganisms secrete mucus to form biofilms, which capture corrosive ions and enrich them in the attached area to produce differential oxygen concentrations, thereby forming an oxygen concentration cell, which causes corrosion of metal materials.
Because microorganisms reproduce quickly and have a wide range of impact, they have attracted great attention from the marine, oil and gas industries. During the transportation and storage of some oil, some microorganisms can directly decompose and consume the oil, while producing toxic acidic substances such as hydrogen sulfide. This not only causes the quality of the oil to deteriorate, but also corrodes equipment such as pipelines, storage tanks and ships.
To prevent microbial corrosion, the following preventive measures can be taken:
Use antibacterial agents: Add bactericides and antibacterial agents to control the growth and reproduction of microorganisms.
Surface cleaning: Regularly clean the sediment and biofilm on the surface of pipes and equipment to avoid microbial accumulation.
Material selection: Choose highly corrosion-resistant materials.
Environmental monitoring: real-time monitoring of microbial activity and corrosion conditions.
Erosion-Corrosion
Erosion-corrosion is a combination of mechanical wear and chemical corrosion, usually caused by mechanical wear caused by the relative movement between the corrosive fluid and the metal surface. In this case, the metal surface is not only eroded by chemical corrosion, but also mechanically worn by fast-flowing liquids or particles, which exacerbates the corrosion.

Erosion corrosion is directional, and the most serious corrosion usually occurs in areas where fluid movement is most intense. This type of corrosion is very likely to occur on the inner walls of some metal pipes that transport liquids, pump impellers, heat exchanger pipes, and other high-velocity areas. As the fluid moves, the protective layer on the metal surface will gradually be washed away, exposing the metal, and then corrosion begins.
The corrosion process forms various pits (circular, oval, and long), which slowly penetrate the metal vertically from the inside, eventually causing the metal wall to become thinner or even perforated.
To prevent erosion and corrosion, you can take the following measures to prevent it::
Design reasonable pipe bending radius and flow velocity to avoid turbulence and drastic flow changes.
Choose alloy materials with higher corrosion resistance or other materials.
Reduce the concentration of suspended particles in liquids.
Regularly check the use of equipment and clean up sediment and worn parts in time.
Fretting Corrosion
Friction corrosion is a special type of local corrosion. It usually occurs in areas where micro-motion wear occurs between two pieces of metal due to vibration or slight sliding. When the oxide film or coating of the metal is damaged due to repeated friction, the internal metal will be exposed to the external environment. The exposed metal is likely to be corroded and oxidized. Coupled with the effect of mechanical wear, the corrosion will continue to worsen and eventually become scrapped. This type of corrosion is most common in some closely contacted links, such as bolt joints, rivet joints, bearings and gears.
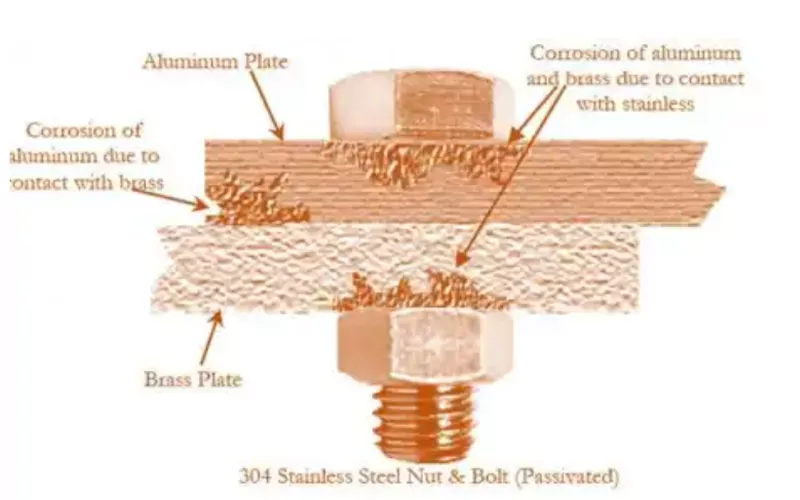
Friction corrosion has some distinctive characteristics:
- Accumulation of corrosive substances: During the fretting process, the oxide debris generated is likely to accumulate on the metal surface.
- Roughening of the surface: More obvious marks will appear in severely damaged areas, and the surface will become rough and even have pits.
- Local strength reduction: Long-term friction and corrosion will greatly weaken the mechanical properties of the metal, leading to fatigue and thus fracture.
You can prevent this corrosion by:
- Apply protective coating: Apply wear-resistant coating and anti-corrosion coating on the contact surface, such as polytetrafluoroethylene (PTFE) and ceramic coating.
- The coating can effectively isolate the metal from the external environment.
- Lubrication treatment: Use appropriate lubricating oil to reduce damage caused by friction
- Optimize contact design: Reduce the relative movement of the contact surface, such as increasing the clamping force.
- Material optimization: Select a combination of metals with a large difference in hardness to reduce surface adhesion and wear.
- Improve environmental conditions: Try to avoid being in a humid environment to aggravate corrosion.
Corrosion Resistant Materials Selection Guide
After knowing the various types of corrosion, are you interested in which materials are corrosion resistant? Here are several major types of corrosion resistant materials and their application areas:
Metal Materials
316L stainless steel:
- Excellent resistance to pitting and crevice corrosion
- Contains 2-3% molybdenum, which can effectively improve resistance to seawater corrosion
Mainly used in chemical equipment, marine engineering and medical equipment. It should be noted that its operating temperature range is about -196℃ to 800℃.
Titanium and titanium alloys:
- Higher strength and corrosion resistance
- Better performance than other metals in chloride environments
Mainly used in aviation, chemical industry and seawater desalination. Although the cost is high, the service life is long.
Hastelloy
- Excellent resistance to high temperature corrosion and stress corrosion cracking
- Can remain stable in strong acid and strong alkali environments.
Mainly used in some high temperature and high pressure equipment, very suitable for extreme corrosive environments
Advanced ceramic materials
Silicon nitride (Si3N4)
- Excellent high temperature resistance and chemical corrosion resistance
- Excellent thermal shock resistance, suitable for some environments with rapid temperature changes
Mainly used in some mechanical bearings, cutting tools, etc., and can be used in high temperature corrosion environments above 850℃
Alumina (Al2O3)
- Excellent chemical corrosion resistance and electrical insulation
- Extremely high hardness and strong wear resistance
Mainly used in some chemical equipment linings, pump bodies, seals, circuit substrates and insulating parts, etc.
Extended reading: Alumina materials
Silicon carbide (SiC)
- High temperature resistance (up to 1600°C) and excellent corrosion resistance
- Excellent thermal conductivity and low thermal expansion coefficient
Mainly used in heat exchange systems, mechanical seals, nozzles, etc., very suitable for high temperature corrosive gas environments
Aluminum nitride (AlN)
- Excellent thermal conductivity and electrical insulation performance
- Good corrosion resistance and chemical stability
Mainly used in electronic packaging and heat dissipation substrates, suitable for some corrosive environments that require high heat dissipation performance.
Composite Materials
Carbon fiber composite material (CFRP)
- High strength-to-weight ratio and superior corrosion resistance
- No electrochemical corrosion
Mainly used in building reinforcement, sports equipment and aviation structural parts, the price is very high, but the performance is very superior
Glass fiber reinforced plastic (FRP)
- Good chemical corrosion resistance
- Lightweight and high strength
Mainly used in storage tanks, pipelines, and building components, it is relatively economical and suitable to replace traditional metal materials.
Coating materials
ceramic coating
- Can provide excellent wear and corrosion resistance protection
- Can be applied to the surface of various substrates
Mainly used in pump body walls, valves, and pipe linings, which can help you significantly extend the service life of your equipment.
Epoxy resin coating
- Excellent adhesion and chemical stability
- Convenient construction
Mainly used for the inner wall of storage tanks, floors, and steel structure protection. The cost is low, but regular inspection and maintenance are required
Material corrosion resistance grade reference table
(Grading criteria: 5 points are the highest and 1 point is the lowest) For your reference only
|
Material Type |
Chemical corrosion |
Seawater Corrosion |
High temperature corrosion |
Stress corrosion |
Comprehensive cost |
Count |
|
Silicon Nitride Ceramics |
5 |
5 |
5 |
5 |
2 |
22 |
|
Silicon Carbide Ceramics |
5 |
5 |
5 |
5 |
2 |
22 |
|
Titanium and titanium alloys |
5 |
5 |
4 |
4 |
2 |
20 |
|
Hastelloy |
5 |
4 |
5 |
4 |
5 |
20 |
|
Alumina ceramics |
4 |
5 |
4 |
5 |
3 |
21 |
|
316L Stainless Steel |
4 |
3 |
3 |
3 |
4 |
17 |
|
Carbon fiber composites |
4 |
4 |
3 |
4 |
2 |
17 |
|
Fiberglass |
3 |
4 |
2 |
3 |
4 |
16 |
|
Ordinary aluminum alloy |
3 |
2 |
2 |
2 |
5 |
14 |
|
Carbon steel + protective coating |
2 |
2 |
2 |
2 |
5 |
13 |
Your best material choice for acidic environment:
1. Silicon nitride ceramics
2. Hastelloy
3. Silicon carbide ceramics
4. 316L stainless steel
5. Alumina ceramics
Your best material choice for alkaline environment:
1. Alumina ceramics
2. Silicon carbide ceramics
3. Titanium alloy
4. 316L stainless steel
5. FRP
Your best material choice for marine environment:
1. Titanium alloy
2. Silicon nitride ceramics
3. Silicon carbide ceramics
4. Hastelloy
5. FRP
Your best material choice for high temperature environment:
1. Silicon carbide ceramic (up to 1600℃)
2. Silicon nitride ceramic (up to 1400℃)
3. Alumina ceramic (up to 1700℃)
4. Hastelloy (up to 1200℃)
5. Titanium alloy (up to 600℃)
Material with the highest cost-effectiveness:
1. 316L stainless steel
2. Fiberglass
3. Carbon steel + protective coating
4. Ordinary aluminum alloy
5. Alumina ceramic
Things you need to pay attention to:
The above ratings are for your reference only and are only applicable to general application environments
The cost includes initial investment and maintenance costs
Conclusion
Understanding the mechanism of corrosion is crucial for your prevention and maintenance. Thank you for reading this article and hope it can help you.