Zirconium dioxide is often known as ceramic steel. It is a tough technical ceramic used in variety of applications. Zirconia, ZrO2 offers good resistance to wear and tear. It also possesses good thermal stability. However, Zirconia ceramic is often stabilized to minimize the behavioral changes with respect to heat loads.
Define Zirconium? How is Zirconia different from Zirconium?
Before diving more in detail into the stabilized forms of Zirconia. It is important to know what is Zirconia, Zirconium, Zircon and Zirconia ceramic. Let’s explore more in detail about these in the ensuing paragraph.
What is Zirconium?
Zirconium is a silvery grey coloured metal produced generally from Zircon. It is found abundantly in earth’s crust either in silicate form in Zircon or very less in baddeleyite. Zr melting point is 1855 degC, Zr density is 6.51 g/cm3 and it has an atomic number around 40. Zirconium metal is the 20th most abundant metal found naturally.
What is Zirconia, ZrO2?
On the other hand, Zirconia Ceramic is one among the most studied ceramic material today. It is often obtained naturally from its ore baddeleyite. Synthetic Zirconia material can also be prepared by melting Zircon (Zirconium silicate) at very high temperature. Zirconium dioxide, ZrO2 generally holds monoclinic structure under room temperature.

The properties of ZrO2
Knowing about the properties of ZrO2 ceramic is very important when it comes to understanding the context of using the ceramic. Let us have a look at the properties of ZrO2
General Properties
|
Chemical Formula and Color |
ZrO2, Color – White |
|
Density of Zirconia |
5.7 g/cm3 |
|
Zirconia melting point and boiling point |
2715 degC and 4300 degC |
|
Zirconia hardness |
5500- 16000 MPa |
|
Thermal conductivity of zirconium oxide |
2 – 3 W/Mk |
|
Structure of Zirconia |
The three phases are Monoclinic (< 1170 degC), Tetrahedral (1170 – 2370 degC) and Cubic (> 2370 degC) |
Other Properties
|
Reactivity |
It is chemically unreactive. When blended with other oxides they tend to get stabilized |
|
Electric Conductivity |
They are insulators |
|
Solubility |
ZrO2 is slowly attacked by HF and H2SO4 |
|
Enthalpy |
Molar Enthalpy of ZrO2 is 50.3 J/Kmol and the Standard enthalpy of formation is 1080 KJ/mol |
What is stabilized zirconia?
Well, before getting to know about stabilized Zirconia. It is important to understand the behaviour of Zirconia under varying heat load. This will give us an idea of why there has been an advancement in stabilizing this Ceramic. In short stabilization of a ceramic is a matter of preserving its structural integrity.
As mentioned ZrO2 undergoes phase transition under varying temperature load. For instance, a temperature change from 1173 degC to 2370 degC make ZrO2 transform from monoclinic to tetrahedral structure. Further when the temperature reaches 2690 degC, ZrO2 becomes cubic. A later rise in temperature causes ZrO2 to melt.
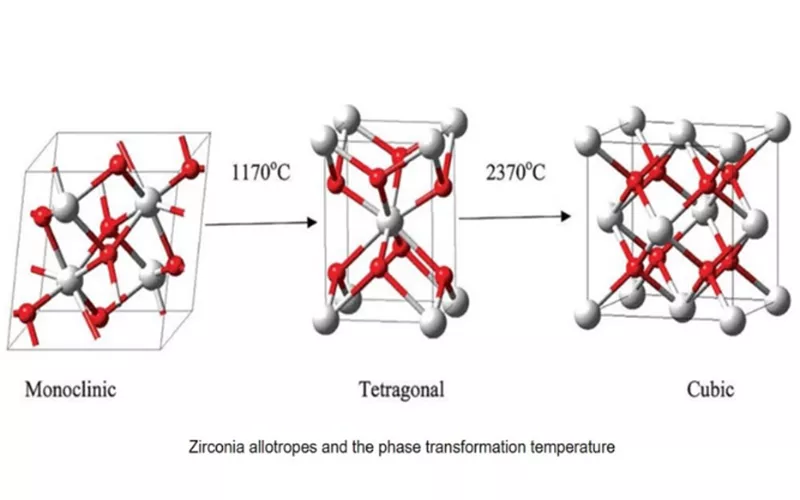
Generally, phase transition also indicates a volume change under which Zirconia ceramic cracks and deforms. Stabilization stops such unfavorable changes happening in the ceramic structure. Hence Zirconia is often stabilized.
Types of Stabilized Zirconia
So, we have understood why stabilization is required when it comes to Zirconia ceramic. Now let us check the different types of stabilized zirconia in detail.
Yttria Stabilized Zirconia (Y2O3.ZrO2)

Yttrium stabilized zirconia is formed by stabilizing cubic Zirconia at room temperature by adding Yttrium Oxide (Y2O3). Stabilization initiates the substitution of Zr4+ with larger ions of Yttrium Y3+ in the crystal lattice. Before stabilizing Zirconium, dioxide will have monoclinic, cubic and tetragonal phases. However once stabilized Yttrium Zirconia (YSZ) will only have cubic structure.
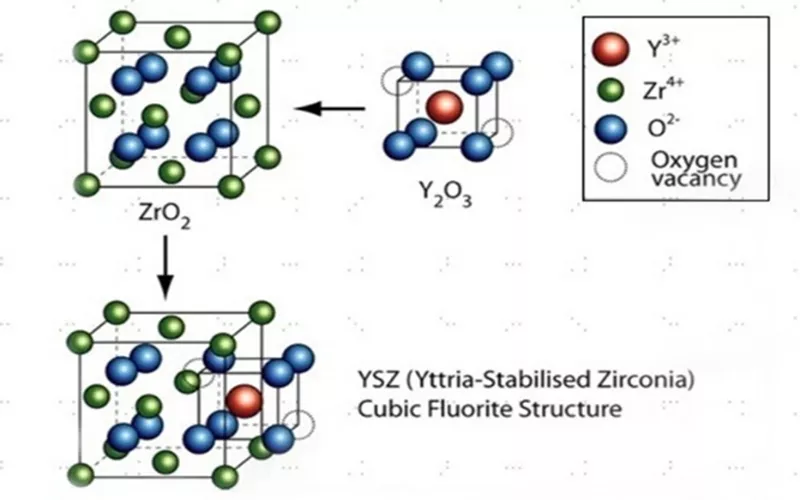
Yttrium stabilized Zirconia properties
- Yttria-stabilized zirconia density is around 6 g/cc. The moderate density of YSZ makes sure there is no excessive weight on any material where it is a part of
- Yttria stabilized zirconia thermal conductivity is at a value of 2.9 W/mK at a temperature of 20 degC.
- Yttrium Zirconia offer a Rockwell hardness of around 85 which is attributed to the best mechanical properties of the ceramic.
Advantages of Yttria (Y2O3) stabilized (ZrO2) Zirconia
The advantages of Yttrium Zirconia are listed below:
- yttria stabilized zirconia ysz offer extreme strength
- The ceramic YZS has high temperature resistance, often used as refractory and used as a thermal barrier coating
- They are corrosion resistant in nature
- YSZ doesn’t undergo rusting and can be used where there are oxidation chances.
Applications of Yttria Stabilized Zirconia (YSZ)
Dentistry
Yttria stabilized Zirconia (YSZ) is extremely hard, biocompatible and is chemically inert. Yttria stabilized Zirconia dental applications include crown, bridges and implants. As we all know our mouth has a moist environment, since Yttrium Zirconia doesn’t corrode. They function as better dental material.
Refractory and thermal coating

The heat resistance of Yttria stabilized Zirconia makes it an ideal refractory. YSZ is also used as a thermal insulation material for insulating hot objects and as coating material on engine surface.
Fuels cells
Yttria stabilized Zirconia (YSZ) serves as electrolyte in solid-state fuel cells. While deploying YSZ as a functioning electrolyte, the selective conductivity of oxygen ion is what is overlooked. The temperature of operation is generally around 800 – 1000 degC.
Other usages
The other uses of Yttrium ceramic are, they form a component in cement manufacture due to its durability. The hardness makes them an ideal material for grinding balls .

Magnesia Stabilized Zirconia (MSZ)
MSZ in comparison with Yttria stabilized Zirconia (YSZ) has less thermal conductivity and more stability in moist conditions. They are wearing resistant, extremely tough and are chemically inert. In structure, Magnesia stabilized Zirconia has tetragonal precipitates with cubic grains.

Properties of Magnesium stabilized Zirconia
- The temperature of operation of Magnesia stabilized Zirconia is above 220 degC
- The prime features of MSZ are high thermal stability, less thermal conductivity and Wear and tear resistance
- MSZ are resistant to acids and base
The major applications of MSZ
- Magnesia stabilized Zirconia are used to manufacture structural or technical ceramics
- They make as ideal material for precision valve components, Pump sleeves and pistons
- Magnesia stabilized Zirconia is used in the manufacture of solid oxide fuel components
- MSZ is used in Tube forming applications
Calcia Stabilized Zirconia (CSZ)

Calcia Stabilized Zirconia (CSZ) as the name says here the stabilizer is calcium oxide. Calcia Stabilized Zirconia (CSZ) has a melting point of 2700 degC with a density of 5.6 g/cm3. They are often known for their high thermal stability and offer extreme shock resistance. The popular applications of Calcia Stabilized Zirconia (CSZ) include Coating for ensuring wear resistance, as a refractory and as thermal barriers.
Ceria Stabilized Zirconia (CSZ)
Ceria Stabilized Zirconia (CSZ) uses Ceria to stabilize the Zirconia in its crystal lattice. They are chemically resistant and are basically used for high temperatures applications. Since the addition of ceria enhances the conductivity of Oxygen, they are used as solid electrolytes in fuel cells. Ceria Stabilized Zirconia (CSZ) also function as Oxygen sensors.

Ceria stabilized Zirconia density is 6.6 g/cm3 with a purity of around 99 %. The applications of CSZ includes high stability grinding materials in paints and related industry. They are also used as catalysts in exhaust systems in automobile industry.
Alumina Stabilized Zirconia (ASZ)
ASZ is made from combining high quality alumina in ZrO2 crystal lattice. This has an alumina percent range from 10 % to 50% depending upon the grade produced. Alumina Stabilized Zirconia offer high fracture toughness and great strength. ASZ are used in automotive applications, aerospace and medical industry.
ASZ is also used for cutting tool manufacture. They are deployed in applications which require high performing ceramic counterparts. The stabilization with Alumina helps it function at harsh conditions. The density of ASZ is around 3.7 – 3.8 g/cc.
Comparing stabilized Zirconia
So, we had a brief idea of why stabilized zirconia and what are the prominent types. Here’s a quick comparison chart having different types of Zirconia.
|
Type of Ceramic |
Properties of Ceramic |
YSZ |
MGZ |
CSZ |
|
Mechanical Properties |
Resistance to wear and tear (MPa^1/2) |
High |
Moderate – High |
Moderate |
|
Strength |
900 – 1200 MPa |
500 – 900 MPa |
200-800 MPa |
|
|
Toughness |
2-5 |
3-10 |
5-15 |
|
|
Electrical Properties |
Thermal conductivity |
2-3 W/mK |
2-4 W/mK |
2-4 W/mK |
|
Coefficient of thermal expansion (10^-6/K) |
10-11 |
9-11 |
10-12 |
|
|
Thermal stability |
< 2700 degC |
< 2500 degC |
< 2400 degC |
|
|
Biocompatibility |
|
High |
Moderate – High |
Moderate – High |
Partially Stabilized Zirconia and Fully stabilized Zirconia
Partially stabilized Zirconia (PSZ) are of different types. It can be Magnesium stabilized, Calcia stabilized or Yttria stabilized. The Zirconia with Magnesia would have approximately 10 % MgO. Magnesia based Partially stabilized Zirconia are extremely tough and works at higher temperature.

Yttria stabilized Zirconia with 4% - 10 % of Yttrium is generally referred as Partially stabilized Zirconia. Whereas the one with 8% of Yttrium and 8-9% Y2O3 doped with ZrO2 is termed as fully stabilized Zirconia. For applications requiring extreme toughness engineers often use PSZ over fully stabilized.
Bottom Line
The advancement in material technology always guarantees the best material for every application. Functionality is the key when it comes to choosing new gen ceramic for desired application. Stabilization ensures stable performance at desired temperature. When it comes to adaptability, of no doubt stabilized Zirconia outperforms the conventional.

