Aluminum nitride is commonly known as a miracle thermal conductor for a few reasons. You’re talking about a futuristic material whose potential seems majorly unexplored. The world had slept on the compound’s potential for more than a century since its first discovery. Thankfully, research has helped us get this unique material’s low-hanging fruits.
Aluminum nitride-based devices are already revolutionizing power, nanotechnology microchips, steel manufacture, and RF applications. With more research, the trend can only go up.
What is Aluminum Nitride?
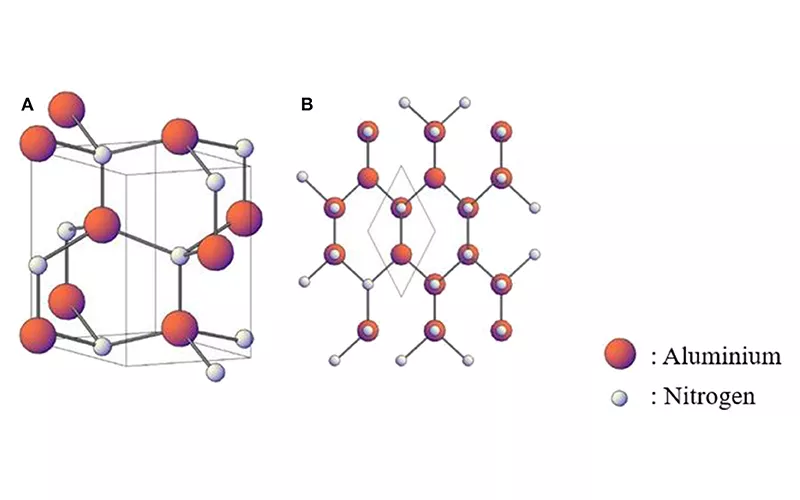
Aluminum nitride is an ionic compound formed by combining aluminum, a metal, and nitrogen, a non-metal. The compound forms through a transfer of electrons from the metal to the non-metal. Aluminum gives its three outer electrons to nitrogen, forming a stable compound. The material has a wurtzite phase, hence its wide band gap semi-conductivity.
Aluminum Nitride Lewis Structure
Aluminum is in group 13 of the periodic table, while nitrogen is in group 15. Meaning that aluminum has 3 covalent (free) electrons, whereas nitrogen has 5. As a non-metal in an ionic compound, nitrogen will need 8 electrons in its outer shell. With that, it will form a stable compound that is similar to noble gases.
Since aluminum has the exact number of electrons nitrogen needs, they combine in a 1:1 ratio. Meaning, only one aluminum and one nitrogen atom are needed to form aluminum nitride (AlN).
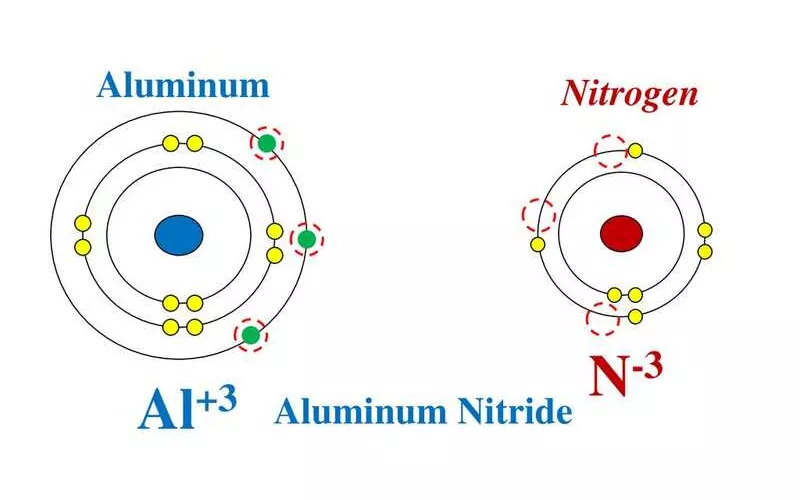
Since nitrogen gets three free electrons from aluminum, it has a 3-ve ionic charge (N3-). Aluminum will have a 3+ charge for giving out its 3 outer electrons (Al3+). The Lewis structure for the compound is as shown below:
AlN Dielectric Constant
Before getting to the dielectric constant concept, you want to understand aluminum nitride as a dielectric material. We all know AlN is an electrical insulating ceramic. But what you may not know is its massive applications in piezoelectronics, microelectronics, etc, as a dielectric material.
So, what about aluminum nitride makes it a dielectric material? What does that mean in the first place?
The word “dielectric” combines two Greek root words, “dia” and “electric.” Dia means passing through, whereas electric means electric field. Hence, the root meaning of dielectric is a material that allows electric fields to pass through it.
An electric field (E field) is a region that surrounds electrically charged particles. If you place a charge, say p in an electric field, it will experience a force that equals the strength of the field multiplied by p, that is F = pE.
When a metal conductor is placed in an electric field, the electrons within it are free to move. The positive charges will move to one end of the piece of metal while the negative charges will move to the opposite side. Hence, you’ll have an electric dipole, creating a new electric field (E’) that opposes the original electric field(E).
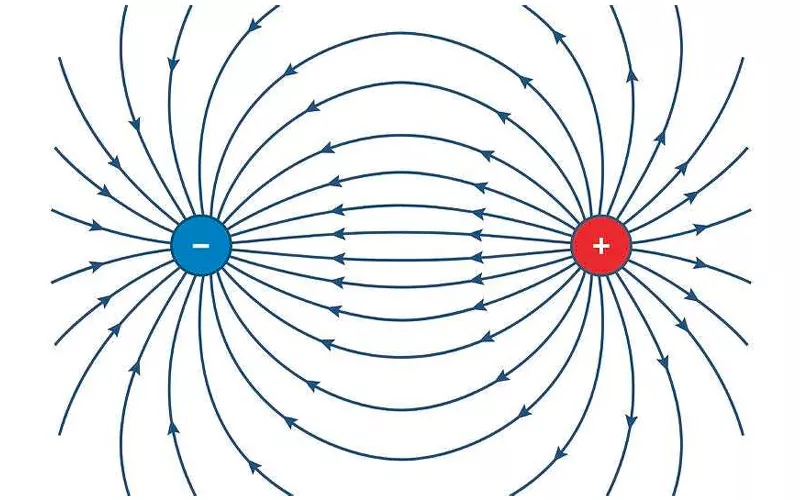
The new electric field continues to form until the charges stop moving and an equilibrium is achieved. Using the resultant field formula, Er = E - E’, you want to find the difference between the original electric field and the new field. Case in point, E - E’ gives zero since the two forces are equal.
Since the resultant field is zero, we conclude that an electrical conductor inhibits electric fields.
As previously mentioned, aluminum nitride, as a dielectric material, is an insulator. When exposed to an electric field, the charges within a dielectric material are not free to move or may move a bit. The ability of an atom to get polarized when exposed to an electric field is subject to its atomic structure. Meaning, a good atomic or molecular structure will give a higher dielectric constant.
The aluminum nitride dielectric constant ranges between 8.3 and 9.3. This indicates the amount of energy stored within aluminum nitride in an electric field. You may want to know how much of the energy stored can be converted into heat and how it reacts to high-temperature conditions. Aluminum nitride’s melting point is as high as 2,200 °C or 3,990 °F. Thus, it decomposes at 1,800 °C (3,270 °F) in a vacuum.
Aluminum Nitride Chemical Properties
The chemical formula for aluminum nitride is AlN, with Al standing for Aluminum and N for Nitride. Aluminum nitride is often confused with aluminum nitrate. While AlN is a form of aluminum nitrate, the two compounds are very different. AlN has a -3 oxidation state, whereas aluminum nitrate is an ester of nitric acid. Besides, the latter compound’s chemical formula is Al(NO₃)₃
Synthesis of Aluminum Nitride
Aluminum nitride is formed through two processes. One includes direct nitridation of aluminum while the other depends on a few factors. The second process involves carbothermal reduction of aluminum oxide. AlN dissociates at temperatures above 2,500 °C. As the material has a 3.26 g.cm-3 density, it dissociates instead of melting above the said temperature.
Sintering is also achievable if you use liquid-forming additives such as CaO or Y2O3 . A few processing methods are used to form different aluminum nitride parts such as dry pressing and cold isostatic pressing. Other processing methods would include ceramic injection molding, precision machining, tape casting, and low-pressure injection molding.

Aluminum nitride is attacked by strong acids and alkali grains. However, it’s resistant to attacks by molten metals like lithium and copper, and molten salts such as cryolite and chloride. Besides, its powder form is easily hydrolyzed by water and humidity. With high volume resistivity, AlN demonstrates high thermal conductivity for a ceramic material and high dielectric strength.
Aluminum Nitride Thermal and Electrical Properties
Aluminum nitride is a remarkable material for its characteristic high thermal conductivity. It also demonstrates high electrical conductivity and is an excellent electrical insulator. These combined with high volume resistivity make AlN a sought-after material in microelectronics for use as a substrate.
Speaking of thermal conductivity, AlN comes second after beryllia. However, it has a higher thermal conductivity than that of copper at moderate temperatures (that is ~200 °C).
AlN is up to task for microelectronic components that require high-volume and resistivity. In microelectronics, you have better cooling substrates than conventional ceramic substrates. Hence they are applied as heat sinks and heat carriers.
In telecommunications, aluminum nitride is used in the manufacture of RF filters for telecommunication gadgets. It also applies as an insulator in clamp rings, lasers, chiplets, microwave device packaging, etc. AlN is stable in carbon and hydrogen and carbondioxide atmospheres of up to 980°C. The material also applies in deep ultra violet optoelectronics.
Its wide band gap of this highly conductive material gives aluminum nitride an upper hand in optoelectronics.
|
Property |
Metric |
|
Density |
3.26gm/cc |
|
Porosity |
0% |
|
Fracture toughness |
2.6MPa•m1/2 |
|
Compressive strength |
2100MPa |
|
Flexural strength |
320MPa |
|
Hardness |
1100Kg/mm2 |
|
Thermal conductivity |
140-180W/m•°K |
|
Diekectric strength |
9 @ 1 MHz |
|
Dissipation factor |
0.0003@ 1 MHz |
|
Volume resistivity |
>1014 >10 ohm.com |
Conclusion
Aluminum nitride’s place in tomorrow’s technology is slowly taking shape. The material’s benefits in microelectronics, peizoelectronics, deep ultraviolet optoelectronics are indispensable. Hence the reason to venture into the aluminum nitride adventure to slot a place for yourself in the future.
